Streamline Regulatory Intelligence
Stay updated on guidance changes, assess business impact, and empower stakeholders with insights from InfoDesk.
See how we helped a global pharma company reduce regulatory surveillance time by 53%.
.png?width=200&name=greyscale%20logos%20(1).png)



Cut your regulatory surveillance time to just 30 minutes a week.
Automate change tracking for faster insights
Centralize updates and documents for easy access
Reduce manual processes for more impactful work
Automated regulatory surveillance
Monitor global health authorities and regulatory bodies to ensure you are always up to date. Get alerted about updates, changes, and more from your InfoDesk regulatory intelligence platform.
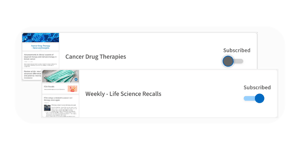
Access curated intelligence from experts
Our team of regulatory experts can monitor and curate intelligence specifically for your use case, so you have time for other strategic initiatives.

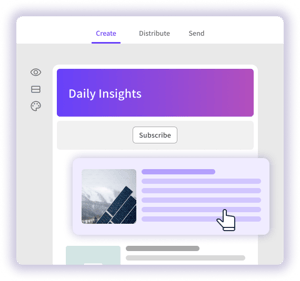
Distribute intelligence at scale
Leverage your regulatory intelligence platform, our team of experts, and best in class deliverables to empower stakeholders with actionable intelligence at their fingertips.
Pharmaceutical leader cuts surveillance time by 53%
In this case study we explore how a global Pharmaceutical manufacturer partnered with InfoDesk to streamline regulatory intelligence with our team of experts and intelligence platform to:
- Save 53% of their time on surveillance
- Distributed regulatory intelligence company wide enabling stakeholders to make informed decisions
- Launched a centralized regulatory intelligence hub

Customer testimonials
Whitepapers, case studies and more
Discover how the Regulatory Intelligence team at a global pharmaceutical organization reduced their manual surveillance time by 53%.
Read More
Discover how InfoDesk enabled a global Quality division to uphold GxP guidelines, streamline regulatory analysis, and align with stakeholders.
Read More
How the Pharmacovigilance team at a global pharmaceutical organization ensured confident auditing and proactive compliance within the Canadian regulatory landscape.
Read More
“InfoDesk exceeded expectations, gaining over 90% satisfaction from end-users.” Find out how a CRO's Regulatory team optimized their workflow.
Read More